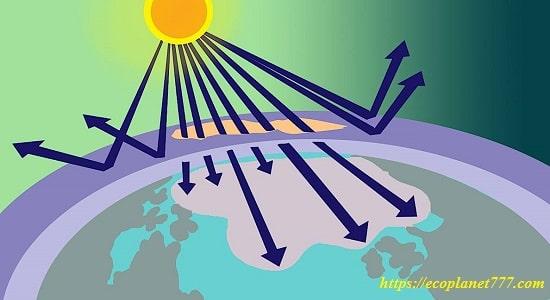
The ozone hole is the thinning of the protective ozone layer in the stratosphere (the upper layer of the earth’s atmosphere). All living things living under the ozone hole are harmed by solar radiation that reaches the surface of the Earth, causing many problems.

The ozone layer protects life on Earth by absorbing ultraviolet radiation, which damages the DNA of plants, animals, and humans and leads to sunburn and skin cancer. Ozone acts as a sunscreen, protecting the Earth’s surface from ultraviolet radiation.
Until 1979, atmospheric ozone concentrations below 220 Dobson Units were not observed by scientists. But in the early 1980s, thanks to a combination of ground-based and satellite measurements, scientists began to realize that the Earth’s natural sunscreen over the South Pole thinned dramatically each spring. This thinning of the ozone layer over Antarctica became known as the ozone hole.
In recent decades, human activity has caused ozone to be destroyed much faster than it can form, thereby creating and expanding the ozone hole.
Ozone can also form at ground level, producing “photochemical smog”; and, because ozone is a toxic gas, there is a health hazard when ozone reaches high levels.
This problem occurs mainly in the summer in cities with heavy traffic, when sunlight interacts with car exhaust gases containing nitrogen oxides.
It’s ironic that human activity is destroying ozone in the upper atmosphere where we need it and creating ground level ozone where we don’t need it!
The appearance of ozone holes
The ozone hole forms every year, due to a sharp decline in total ozone over most of Antarctica for about three months (September-November) in the Southern Hemisphere.
This occurs as a result of the destruction of stratospheric ozone by gases containing chlorine and bromine, the sources of which are mainly human-produced halocarbon gases.
Cause of the ozone hole over Antarctica
The earth’s atmosphere is constantly stirred around the globe by winds. As a result, ozone-depleting gases are mixed throughout the atmosphere, including Antarctica, no matter where they are emitted. Due to the special meteorological conditions in Antarctica, these gases are more effective at destroying the ozone layer than anywhere else.
Each season, the appearance of the ozone hole and its evolution are monitored by satellites and a number of ground observation stations.Ozone hole characteristics, interactive maps, time series, current status and forecast are produced and monitored by the large ozone community through the services of various organizations such as the Copernicus Atmospheric Monitoring Service (CAMS), NASA Ozone Observing Program, NOAA, KNMI, ECC and others .
What is an ozone hole? Essence
If you ask someone at random what an ozone hole is, there’s a good chance they’ve heard of this problem before, but can they explain what it is and why it happens?
Despite being widely known, the problem of ozone holes remains one of the least understood by the public environmental problems.
Scientists use the word “hole” as a metaphor for the area where the ozone concentration falls below the historical threshold of 220 Dobson Units (named after the Dobsonian spectrophotometer used to make the measurements). Using this metaphor, they can describe the size and depth of the hole.
In other words, the ozone hole is the thinning of the protective ozone layer in the stratosphere (the upper layer of the earth’s atmosphere). People, plants and animals living under the ozone hole are harmed by solar radiation that reaches the Earth’s surface, causing health problems ranging from eye damage to skin cancer.
The ozone layer is very important for life on Earth because it has the ability to absorb this dangerous form of UV radiation. It is constantly being formed and destroyed, and its distribution across the planet is uneven and not constant.
The ozone hole. Cause
The ozone hole was formed due to the fact that people polluted the atmosphere with chemicals containing chlorine and bromine. The main chemicals involved are chlorofluorocarbons (CFCs for short), halons and carbon tetrachloride. CFCs have a wide range of uses, including refrigeration, air conditioning, foam packaging and aerosol cans.
The ozone hole actually opened the eyes of the world to such a process as the global impact of human activity on the atmosphere. Scientists have discovered that chlorofluorocarbons (CFCs) — long-lived chemicals that have been used in refrigerators and aerosol cans since the 1930s — have a dark side. In the layer of the atmosphere closest to Earth (the troposphere), CFCs have been circulating for decades without decomposing or reacting with other chemicals. However, when they reached the stratosphere, their behavior changed. In the upper stratosphere (beyond the protection of the ozone layer), ultraviolet radiation caused the breakdown of chlorofluorocarbons to release chlorine, a highly reactive atom that repeatedly catalyzes ozone destruction.
The formation of ozone holes. Where does this phenomenon take place?
The 2020 Antarctic ozone hole has been growing rapidly since mid-August and peaked at approximately 24.8 million square kilometers on September 20, 2020, spreading over much of the Antarctic continent.
It was the longest and one of the biggest and deepest holes since ozone monitoring began 40 years ago.The reason for this phenomenon was a strong, stable and cold polar vortex and very low temperatures in the stratosphere (a layer of the atmosphere at a height of 10 to 50 km). The same meteorological factors also contributed to the formation of the record-breaking ozone hole in the Arctic in 2020.
During the Southern Hemisphere spring season (August-October), the Antarctic ozone hole increases in size, peaking between mid-September and mid-October. As temperatures high in the atmosphere (stratosphere) begin to rise in late spring in the Southern Hemisphere, ozone depletion slows, the polar vortex weakens and finally collapses, and by late December ozone levels return to normal and the hole closes.
The emergence and expansion of ozone holes
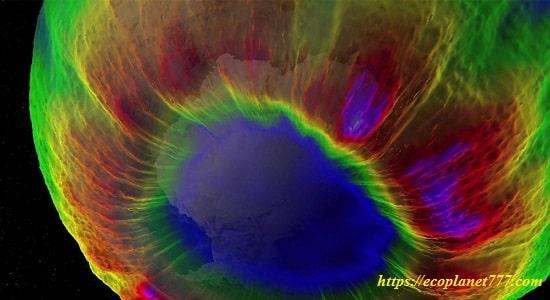
In the early 1980s, the Earth’s natural solar layer over the South Pole began to thin sharply every spring, and an ozone hole began to form there.
From 1980 to early 1990s. The thinning of the ozone layer over Antarctica has grown rapidly in area and depth. The colder conditions of this region result in a larger area and lower ozone values at the center of the hole.
For several years, the minimum concentrations remained at the level of 190, but then the minima quickly deepened: 173 units. in 1982, 154 in 1983, 124 in 1985
By 1991, a new threshold was passed as the ozone concentration dropped below 100 DU. for the first time. Since then, concentrations below 100 have become more common. The deepest ozone hole was formed in 1994, when the concentration dropped to 73 units on September 30th.In the early 21st century, annual ozone holes have stabilized slightly.
Ozone holes in the atmosphere
There are still enough ozone-depleting substances in the atmosphere to cause ozone depletion every year.
Ozone depletion is also directly related to the temperature of the stratosphere, which is the layer of the atmosphere between 10 and 50 km above sea level. This process is due to the fact that polar stratospheric clouds, which play an important role in the chemical destruction of ozone, form only at temperatures below -78°C.
These polar stratospheric clouds contain ice crystals that turn non-reactive compounds into reactive ones, which then rapidly destroy ozone as soon as sunlight becomes available to start chemical reactions. This dependence on polar stratospheric clouds and solar radiation is the main reason why the ozone hole is only observed in late winter/early spring.
Ozone hole over Antarctica
The Earth’s natural sun cover over the South Pole thins sharply every spring. This depletion of ozone over Antarctica is known as the ozone hole.
Usually there are no clouds in the stratosphere, and the reason for this is that there is very little water vapor at this altitude. However, during the austral winter, the air in the stratosphere over Antarctica drops to temperatures below -80°C; enough to form thin clouds. As long as it’s dark, nothing happens; but when spring comes, the Sun’s UV radiation reaches the Antarctic Circle and starts the process of chlorine release and ozone destruction. This continues until the stratospheric clouds disappear due to the warming of the south polar atmosphere as summer approaches.
By summer time, the stratospheric air of lower latitudes is able to penetrate into the polar latitudes and thereby cause the appearance of a denser ozone layer over Antarctica. Consequently, there is a seasonal ozone hole cycle over Antarctica, with the lowest ozone levels recorded in late September and early October.
Although the same processes lead to ozone depletion in the Arctic at the opposite time of the year, the problem is not as severe as in the south because the stratosphere over the Arctic tends not to get as cold as the stratosphere over the Antarctic. Therefore, the formation of stratospheric clouds is not so common and widespread in the Arctic, this is mainly due to differences in the distribution of land and sea between the two regions.
Consequences and influence of ozone holes. Why are they dangerous?

The consequences of the destruction of the ozone layer for humans and the environment are devastating.
Ozone depletion causes an increased level of UV radiation on the Earth’s surface, which is detrimental to human health. Negative effects include the spread of certain skin cancers, eye cataracts, and immunodeficiency conditions.
UV – radiation also causes changes in the growth of food chains and biochemical cycles. Aquatic life just below the surface of the water, which is the backbone of the food chain, is particularly affected by high levels of UV radiation.
UV rays also affect plant growth, reducing agricultural productivity. This can lead to stunted plant growth, smaller leaf size, poor flowering and plant photosynthesis, and reduced crop quality for humans. And reduced plant productivity will in turn affect soil erosion and the carbon cycle.
Plankton and zooplankton are severely affected by UV exposure. They are at the first links of the aquatic food chain. If plankton numbers decrease, this is likely to have far-reaching consequences for all marine life at the bottom of the food chain.
Ecological problem of ozone holes. Global Issues
The main aspect of the ecological problem of the ozone hole is its connection with global warming, that is, climate change as a result of increased greenhouse gas emissions. Both problems are the result of atmospheric pollution, but the causes and consequences of each are very different.
Despite the differences, there are some links between the two problems.For example, CFCs (chlorofluorocarbons) are greenhouse gases and cause ozone depletion, so phasing out CFC production helps fight climate change as well as restore the ozone layer.
Let’s look at the global problems associated with ozone depletion.
The ozone layer protects life from harmful UV radiation, which can cause cancer and slow down plant growth. UV radiation can also penetrate the surface of the ocean, so marine organisms (especially phytoplankton) also suffer from this effect. If humanity continues to destroy the ozone layer, plant photosynthesis will be disrupted, ecosystems will cease to function properly, so it is in our interests to protect and renew ozone to prevent the formation and expansion of new holes.
When the extent of ozone depletion over Antarctica became apparent in 1985, it did not take long for the international community to reach a consensus on what needed to be done. If not for quick political action, ozone-depleting chemicals would continue to build up in the atmosphere, increasing the size of the Antarctic ozone hole, as well as thinning the ozone layer elsewhere in the south, such as New Zealand and Australia.
Ozone holes and the greenhouse effect
A group of greenhouse gases including chlorofluorocarbons (CFCs) are responsible for ozone depletion as they attack and destroy ozone molecules. CFCs are used in aerosols such as hairspray cans, refrigerators, and in the production of foams.
The resulting ozone holes let in harmful ultraviolet radiation and increase the greenhouse effect.
Vehicles also emit large amounts of greenhouse gases, which lead to global warming, the greenhouse effect, and ozone depletion. Therefore, it is necessary to switch to other modes of transport and alternative environmental fuels.
Acid rain
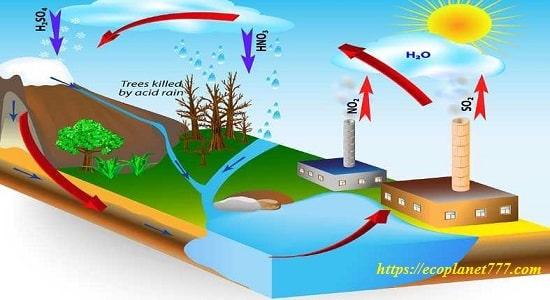
When fossil fuels are burned, oxides of sulfur and nitrogen are emitted into the atmosphere. When sulfur dioxide and nitrogen dioxide mix with water droplets in the atmosphere to form sulfuric and nitric acids, acid rain forms. The winds carry these pollutants for thousands of miles, then they fall to the Earth’s surface in the form of acid rain, which damages the leaves of vegetation, increases the acidity of the soil and water, and kills about 500 people every year.
Buildings and other structures are also affected by acid rain, which causes about $5 billion in property damage every year. The stone elements on the building are crumbling and damaged.
Acid rain dissolves mortar between bricks, makes stone foundations unstable, and destroys ancient buildings and statues made of marble or limestone.
Ozone hole pollution
Wildlife and people experience many of the negative effects of air pollution. Damage to the respiratory system is the most common consequence in humans and animals, but neurological problems and skin irritation are also common.
Plants and crops grow less with prolonged exposure to polluted air. Ozone pollution harms plants by damaging structures called stomata, which are tiny pores on the underside of leaves that allow the plant to “breathe”. Some plant species can protect themselves by temporarily closing their stomata or by producing antioxidants, but others are particularly susceptible to damage.
Between 1980 and 2011, nine billion dollars worth of soybeans and corn were lost in the US to ozone pollution. When acid rain, lead toxicity, and exposure to nitrogen oxides alter soil chemistry, plants are deprived of the nutrients they need to grow and survive. It affects agriculture, forests and pastures.
How to warn? Solution
In 1987, to address the problem of ozone depletion, the international community adopted the Montreal Protocol, an agreement to phase out the production of ozone-depleting chemicals. It was the first international treaty signed by every country in the world and is considered the greatest environmental success in the history of the United Nations.
The aim of the Montreal Protocol is to reduce the production and consumption of ozone-depleting substances in order to reduce their presence in the atmosphere.
Global consumption of these substances has fallen by about 98% since countries started taking action under the Montreal Protocol. As a result, the concentration of the most aggressive types of ozone-depleting substances in the atmosphere decreases and the first signs of the restoration of the ozone layer appear.
Everyone must also take steps to prevent ozone depletion. Avoid the use of pesticides and switch to natural pest control methods instead of using chemicals. Vehicles emit large amounts of greenhouse gases, which lead to global warming as well as ozone depletion. Therefore, the use of vehicles should be kept to a minimum. Most cleaning products contain chemicals that affect the ozone layer. We must replace them with environmentally friendly products. Monitor the operation of air conditioners, as their malfunction leads to the release of CFCs into the atmosphere.
Scientists have already received the first definitive evidence of ozone recovery by observing a 20 percent decrease in ozone depletion during the winter months from 2005 to 2016. Models predict that the Antarctic ozone layer will recover by 2040.
P.S.
If you liked this information and found it useful, please share it on social media. networks with your friends and acquaintances. This is how you support our project “Ecology of Life” and make your contribution to the preservation of the environment!
- Magnetic storms: the sun is testing the planet🌪️ - 13.06.2024
- Why You Should Drink Chicory: Benefits and Harms 🌿 - 09.06.2024
- Innovative Choice: Sproud Milk – Your Ideal Plant-Based Drink 🌱 - 03.06.2024



